Agarose functionalized phosphorus ligand for stabilization of small-sized palladium and copper nanoparticles: efficient heterogeneous catalyst for Sonogashira reaction - ScienceDirect

New palladium (II) complexes containing phosphine‐nitrogen ligands and their use as catalysts in aminocarbonylation reaction - Aranda - 2019 - Applied Organometallic Chemistry - Wiley Online Library

Chiral Bifunctional Phosphine‐Carboxylate Ligands for Palladium(0)‐Catalyzed Enantioselective C−H Arylation - Yang - 2018 - Angewandte Chemie International Edition - Wiley Online Library

Parameterization of phosphine ligands demonstrates enhancement of nickel catalysis via remote steric effects | Nature Chemistry

Synthesis, characterization, coordination chemistry, and luminescence studies of copper, silver, palladium, and platinum complexes with a phosphorus/nitrogen/phosphorus ligand - ScienceDirect
![Synthesis of 3-aryl-2-phosphinoimidazo[1,2- a ]pyridine ligands for use in palladium-catalyzed cross-coupling reactions - RSC Advances (RSC Publishing) DOI:10.1039/C9RA02200G Synthesis of 3-aryl-2-phosphinoimidazo[1,2- a ]pyridine ligands for use in palladium-catalyzed cross-coupling reactions - RSC Advances (RSC Publishing) DOI:10.1039/C9RA02200G](https://pubs.rsc.org/image/article/2019/RA/c9ra02200g/c9ra02200g-s1_hi-res.gif)
Synthesis of 3-aryl-2-phosphinoimidazo[1,2- a ]pyridine ligands for use in palladium-catalyzed cross-coupling reactions - RSC Advances (RSC Publishing) DOI:10.1039/C9RA02200G
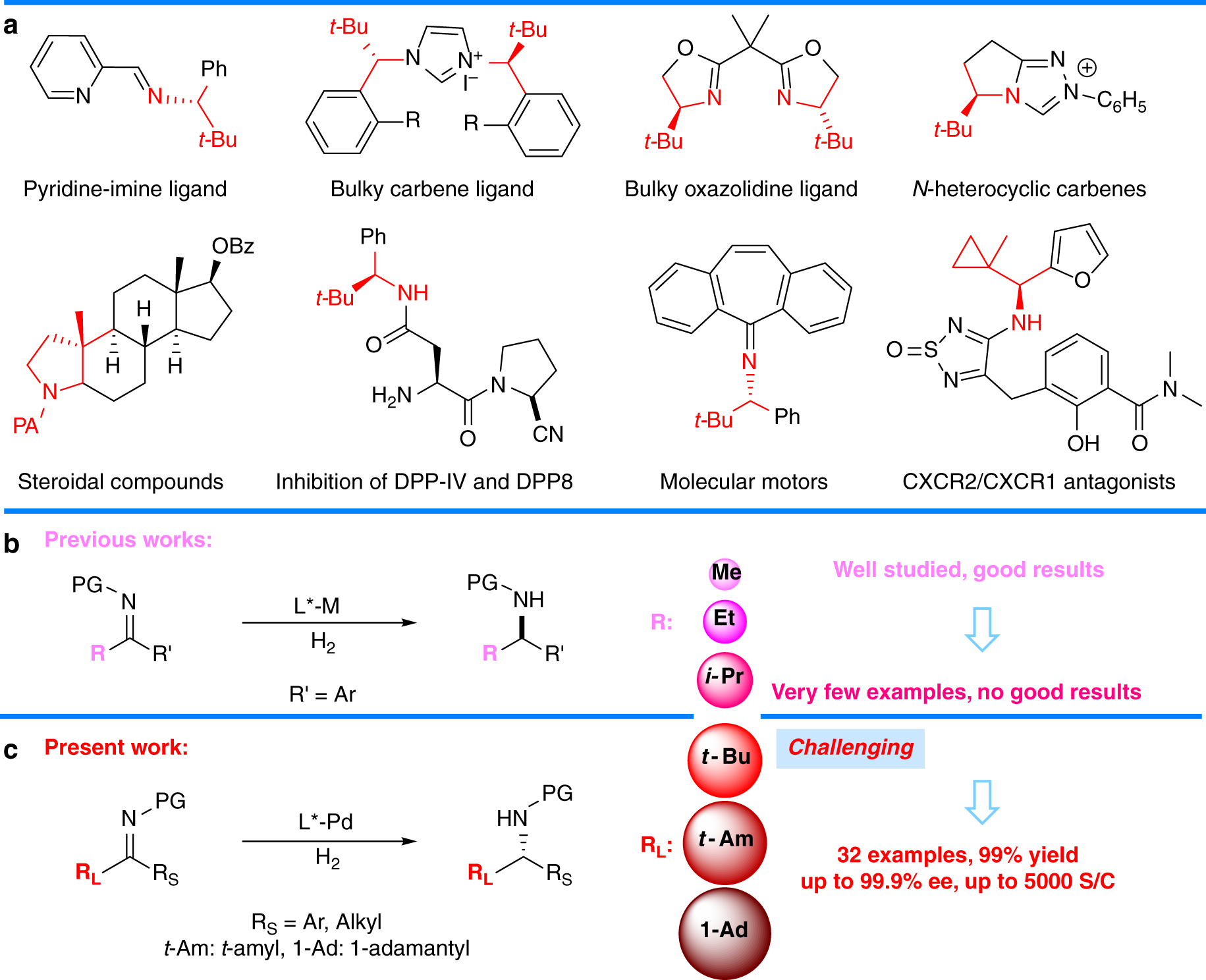
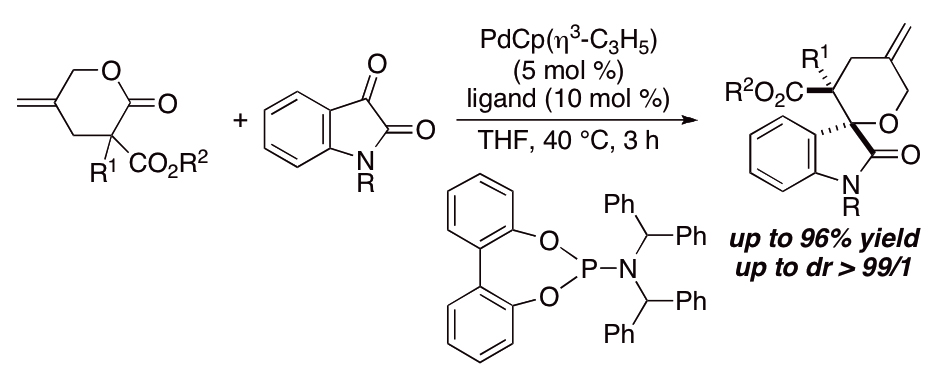
Pd(OAc)2-catalyzed asymmetric hydrogenation of sterically hindered N-tosylimines | Nature Communications

Palladium-catalyzed phosphorus–carbon bond formation: cross-coupling reactions of alkyl phosphinates with aryl, heteroaryl, alkenyl, benzylic, and allylic halides and triflates - ScienceDirect
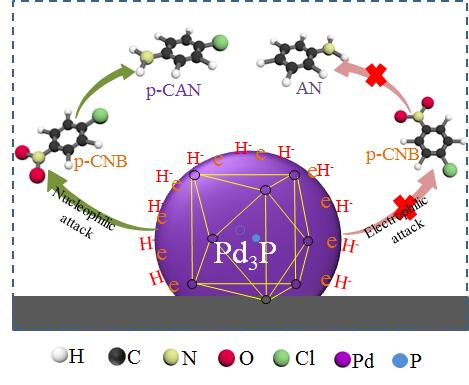
Catalysts | Free Full-Text | Preparation and Catalytic Performance of Metal-Rich Pd Phosphides for the Solvent-Free Selective Hydrogenation of Chloronitrobenzene | HTML
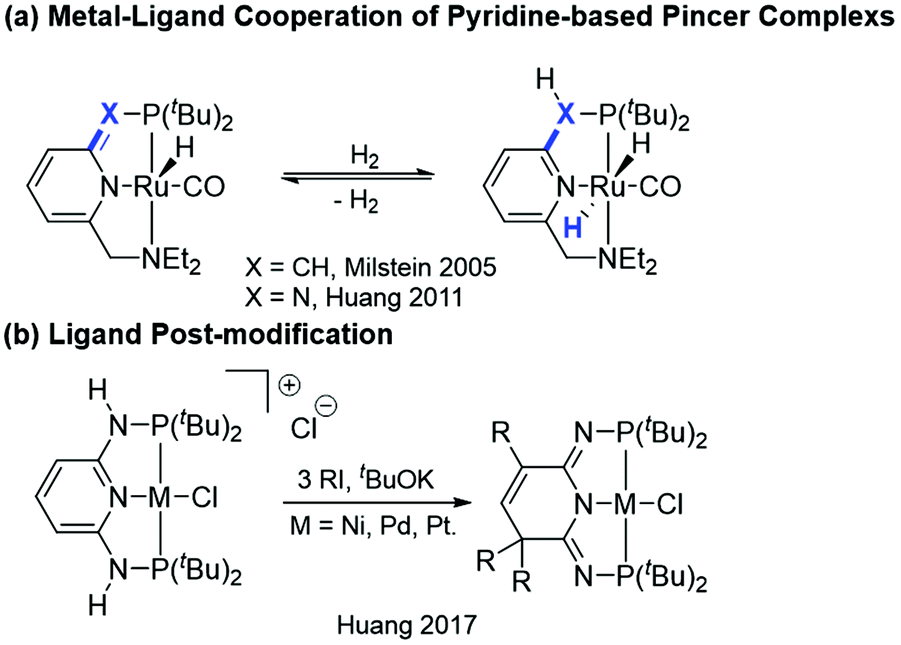
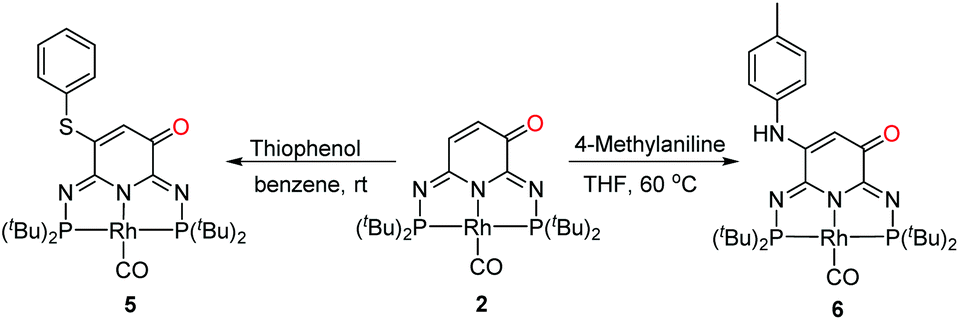
Ligand-centered reactivity of a pseudo-dearomatized phosphorus-nitrogen PN 3 P* rhodium complex towards molecular oxygen at room temperature - Inorganic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C9QI01605H
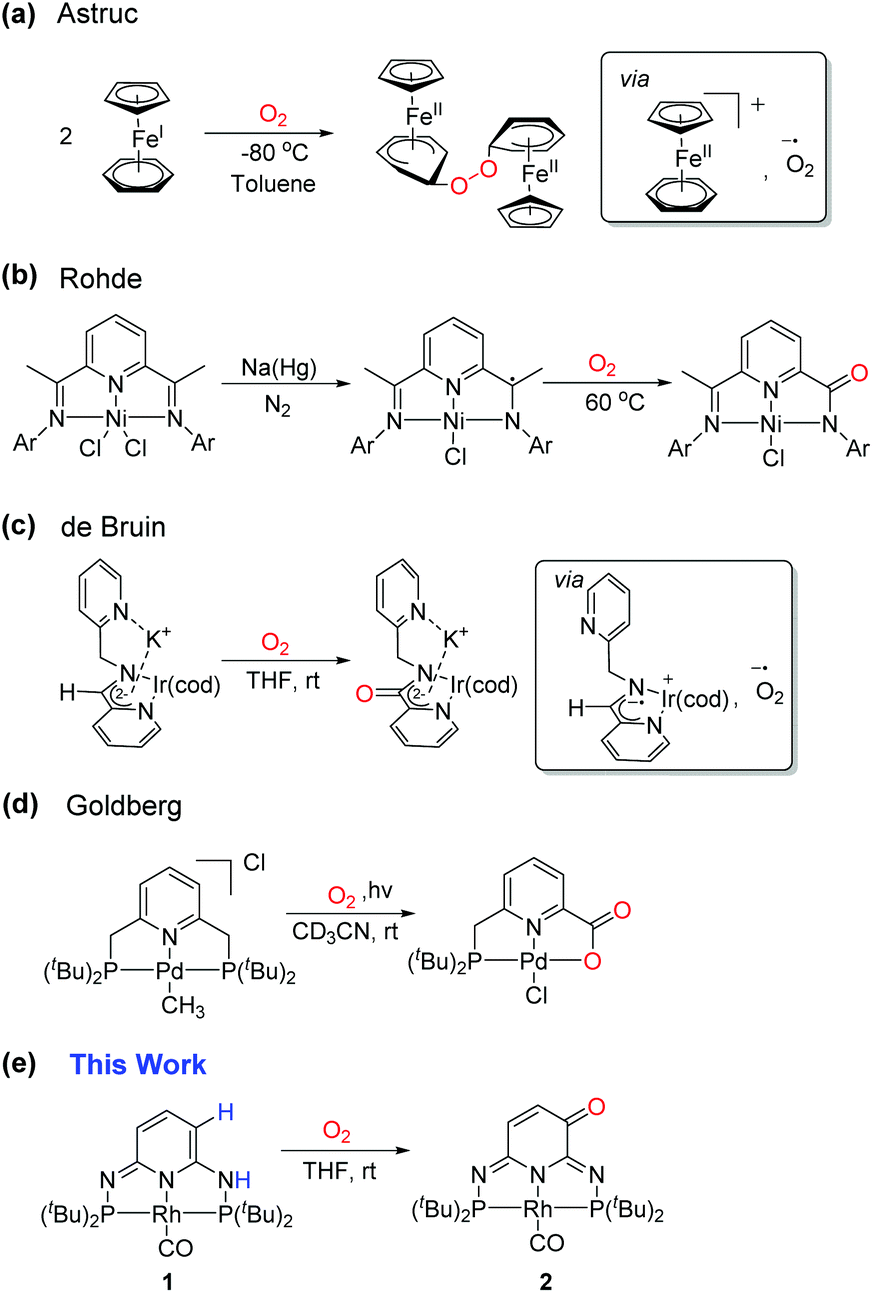
Ligand-centered reactivity of a pseudo-dearomatized phosphorus-nitrogen PN 3 P* rhodium complex towards molecular oxygen at room temperature - Inorganic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C9QI01605H

Cationic palladium(II)–acetylacetonate complexes containing phosphine and aminophosphine ligands and their catalytic activities in telomerization of 1,3-butadiene with methanol - ScienceDirect